Oncology
CRISPR and Cancer: A Path Forward in Oncology
Cancer, one of the leading causes of mortality worldwide, has long been a formidable opponent in the medical field. Traditional treatments such as chemotherapy, radiation, and surgery have improved survival rates, but they often come with significant side effects and are not always effective, particularly against advanced or resistant cancers. In recent years, however, breakthroughs in genetic engineering have opened up new avenues for cancer treatment, offering hope for more effective and personalized therapies. One of the most promising tools in this new era of cancer treatment is CRISPR-Cas9, a powerful technology that allows scientists to edit genes with unprecedented precision.
.png?width=600&height=400&name=unnamed%20(89).png)
Figure 1. Mesothelin-specific CAR T cells attacking a cancer cell. (Credit: Prasad Adusumilli / Memorial Sloan Kettering)
A Path Forward in Oncology
Cancer is fundamentally a genetic disease, characterized by mutations that lead to uncontrolled cell growth and the formation of tumors. These mutations can be inherited, but more commonly, they occur sporadically due to environmental factors, lifestyle, or errors in DNA replication. The ability of CRISPR to target and modify specific genes makes it an ideal tool for addressing the genetic mutations that drive cancer.
Researchers are exploring several ways to use CRISPR in cancer treatment. One approach is to directly edit cancer cells to disrupt the genes that promote tumor growth or to restore the function of genes that suppress tumors. For instance, CRISPR can be used to knock out oncogenes—genes that have the potential to cause cancer when mutated or overexpressed. By inactivating these oncogenes, CRISPR could potentially halt the progression of the disease.
Another promising application is using CRISPR to make cancer cells more susceptible to existing treatments. For example, some cancers develop resistance to chemotherapy by acquiring additional mutations. CRISPR could be employed to reverse these mutations or sensitize cancer cells to drugs, enhancing the effectiveness of treatment.
CRISPR and Immunotherapy: A Powerful Toolbox
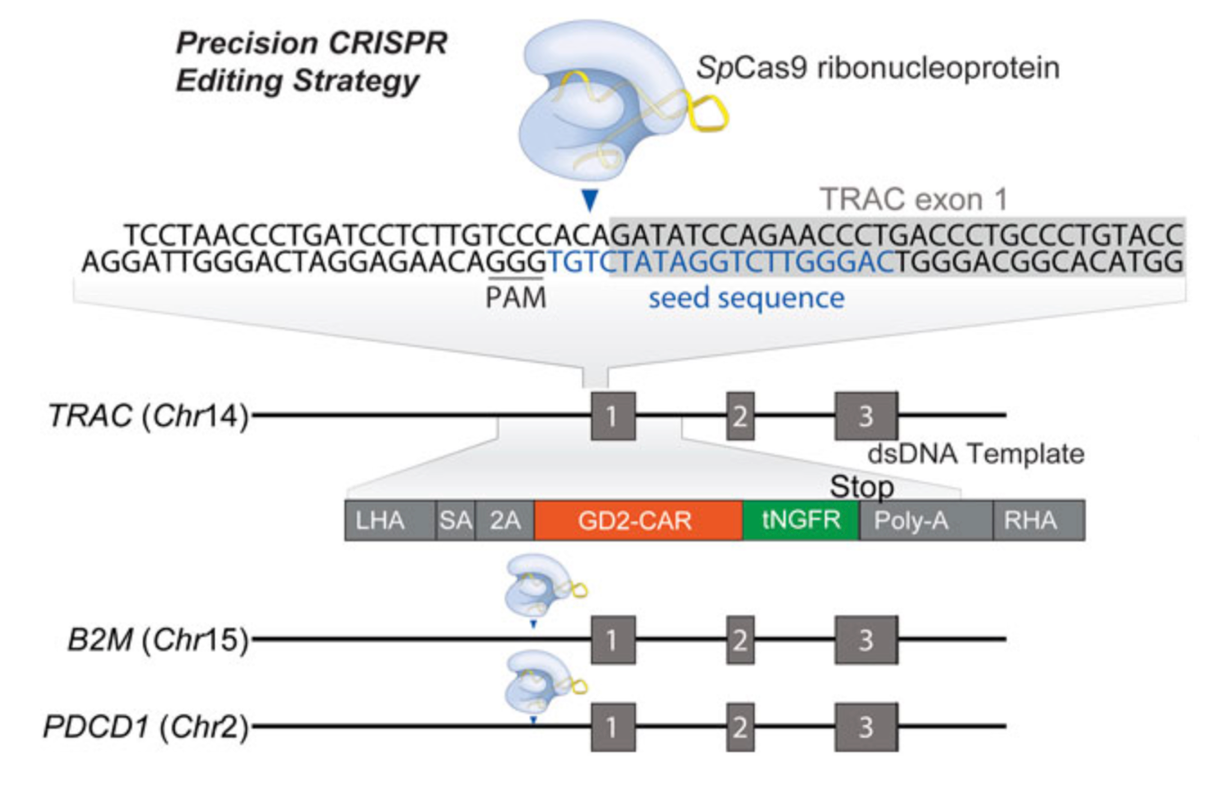
Immunotherapy, which harnesses the body's immune system to fight cancer, has emerged as a groundbreaking approach in oncology. While traditional therapies like chemotherapy and radiation often target cancer cells directly, immunotherapy aims to boost or modify the immune system to recognize and destroy cancer cells more effectively. Among the various forms of immunotherapy, CAR-T cell therapy and T-cell receptor (TCR) therapy have shown remarkable success, particularly in treating blood cancers like leukemia and lymphoma. CRISPR is now being integrated with these therapies to further enhance their efficacy and broaden their applicability.
.png?width=300&height=385&name=unnamed%20(90).png)
Figure 2. CAR-T versus TCR molecular mechanisms. (Source: BioRender)
Chimeric Antigen Receptor T-cell (CAR-T) therapy involves extracting T-cells from a patient's blood, genetically engineering them to express a receptor that recognizes a specific protein on the surface of cancer cells, and then infusing these modified cells back into the patient. The engineered T-cells can then seek out and destroy the cancer cells.
CRISPR is being used to improve CAR-T therapy in several ways. One approach is to enhance the specificity and persistence of CAR-T cells by editing genes that regulate T-cell function and survival. For example, researchers are using CRISPR to knock out the PD-1 gene in CAR-T cells, which is responsible for immune checkpoint inhibition. By preventing PD-1 expression, the engineered T-cells are less likely to be "turned off" by the tumor's immune evasion mechanisms, potentially improving the therapy's effectiveness.
Another innovative application of CRISPR in CAR-T therapy is the development of "universal" CAR-T cells. These are T-cells derived from healthy donors that are genetically modified to prevent rejection by the recipient's immune system. By knocking out genes that contribute to immune rejection, such as the T-cell receptor (TCR) gene, researchers hope to create off-the-shelf CAR-T therapies that are more accessible and cost-effective.
.png?width=500&height=507&name=unnamed%20(91).png)
Figure 3. How CRISPR is changing cancer research and treatment. (Credit National Cancer Institute)
TCR Therapy
T-cell receptor (TCR) therapy is another form of immunotherapy that, like CAR-T therapy, involves genetically engineering T-cells to target cancer cells. However, while CAR-T cells recognize antigens on the surface of cancer cells, TCR therapy targets intracellular proteins presented on the surface by molecules called human leukocyte antigens (HLAs). This allows TCR therapy to target a broader range of cancers, including solid tumors, which have been more challenging to treat with CAR-T therapy.
CRISPR is being employed in TCR therapy to improve the precision and efficacy of these engineered T-cells. For instance, CRISPR can be used to knock out endogenous TCR genes in T-cells before introducing the engineered TCR, which reduces the risk of mispairing between the natural and introduced receptors. This mispairing can lead to off-target effects and reduce the effectiveness of the therapy.
Moreover, CRISPR can be used to enhance the ability of TCR-engineered T-cells to survive and function in the tumor microenvironment, which is often immunosuppressive. By knocking out genes that inhibit T-cell activity or by inserting genes that enhance T-cell function, researchers are working to create TCR therapies that are more potent and durable.
How EditCo is Advancing Cancer Drug Discovery
EditCo’s products have been instrumental in the progress of oncology research for years now. EditCo’s KO and KI immortalized and primary cell offerings enable researchers to study tumor progression, metastasis, and resistance mechanisms. They have also been used in target identification and validation for oncologic therapies. Whether you want to screen multiple genes of interest or target a specific loci, EditCo has the expertise to enable your oncology research.
Intelligent Guide Design

EditCo’s innovative approach to XDel Technology allows efficient gene disruption generation, eliminating the trial and error associated with common guide design strategies. All our reagents are designed to streamline your research, ensuring more accurate and reliable results.
Target Identification & Validation: Finding & Confirming Therapeutic Targets with Confidence

With intelligent guide design strategy, EditCo is able to simply and effectively knockout genes with our Gene Knockout Kits or do high-throughput genomic screening with Arrayed gRNA Libraries, allowing for clear genotype-to-phenotype answers for many genes at scale and quickly.
Using CRISPR to knockout genes at various scales allows researchers to quickly identify potential oncogenic drivers, gene-gene interactions, or drug resistance pathways. The arrayed screening libraries, such as EditCo’s Arrayed gRNA Libraries or unique Engineered Cell Libraries, allow for one gene to be targeted per well across multiwell plates, creating straightforward genotype-phenotype connections with minimal data analysis. Additionally, pre-designed screening libraries can also include cancer-specific gene families like the complete druggable genome, G protein-coupled receptors (GPCRs), and immunology/immuno-oncology genes), custom gene libraries, or even the whole human/mouse genome.
Target validation is the confirmation of identified targets from target screening workflows. Secondary confirmation of genotype-phenotype interactions is critical to ensure confidence in proceeding toward complex functional assays with novel drug targets. Confidence in a functional knockout for a particular gene is necessary to study cancer-specific processes such as the epithelial-mesenchymal transition, where assay sensitivity is critical. Improving the reliability of this step, with either CRISPR knockout reagents, like Gene Knockout Kits, or simply using custom-edited Immortalized Knockout Cells, can make it easier.
Lead Identification and Optimization: Evaluate Therapies, Not Cell Models

Lead validation confirms candidate new molecular entities, biologics, or cell gene therapies for a particular disease indication. In cancer contexts, this step evaluates these therapeutics against model cell lines carrying relevant oncogenic or drug resistant genotypes. The data generated from these experiments is critical for the continued optimization of lead therapeutics. Moreover, certainty in the genotype of challenged cell models or modified cell gene therapies is integral in highly sensitive assays such as transcriptomics. Cell models with a complete phenotypic knockout and defined genetic background, such as EditCo’s Knockout and Knock-in Immortalized Cell Clones, are critical for ensuring sensitivity in evaluating lead therapeutic indications in complex downstream phenotypic assays.
Have more questions? Reach out to us!
More about EditCo and Oncology