Stem Cell Therapy
Stem Cell Therapy and the CRISPR Revolution: A New Frontier in Medicine
Stem cell therapy has long been heralded as one of the most promising frontiers in regenerative medicine. The ability to replace or repair damaged tissues and organs using stem cells holds the potential to transform the treatment of diseases that are currently incurable. Over the past decade, the emergence of CRISPR-Cas9 gene-editing technology has opened new avenues in stem cell therapy, pushing the boundaries of what is possible in regenerative medicine. This article explores the intersection of stem cell therapy and CRISPR, highlighting how this powerful combination is poised to revolutionize medical treatments.
Fig 1. Cluster of human stem cells. (Credit: Allen Institute for Cell Science)
Understanding Stem Cell Therapy
Stem cells are unique in that they have the ability to develop into many different cell types in the body during early life and growth. They also serve as a sort of internal repair system, dividing essentially without limit to replenish other cells as long as the person or animal is still alive. Stem cell therapy leverages these capabilities to replace cells that are damaged or destroyed by disease.
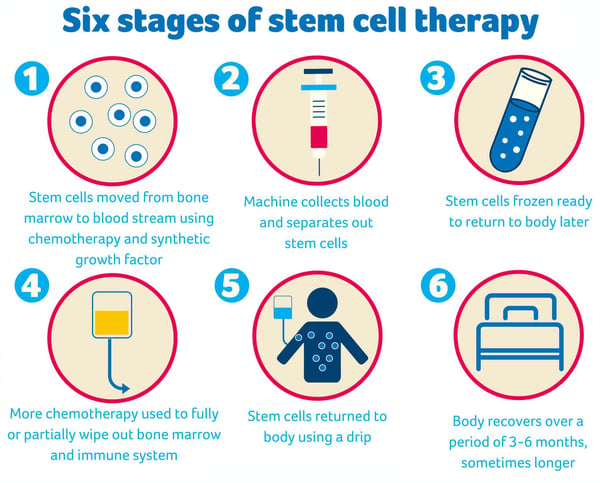
Stem cell therapy has been explored for a variety of conditions, including neurodegenerative diseases like Parkinson’s, spinal cord injuries, diabetes, and heart disease. The potential to regenerate tissue and restore function makes stem cell therapy a tantalizing option for patients facing these devastating conditions. However, despite its potential, stem cell therapy faces several challenges, including immune rejection, limited differentiation, and ethical concerns surrounding the use of embryonic stem cells.
The Role of CRISPR in Stem Cell Therapy
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) is a groundbreaking gene-editing technology that allows scientists to precisely modify DNA within living organisms. By using the Cas9 protein as a pair of molecular scissors, researchers can target specific genes for deletion, insertion, or modification. This precision makes CRISPR a powerful tool for correcting genetic defects, understanding disease mechanisms, and engineering cells with new capabilities.
In the context of stem cell therapy, CRISPR has several transformative applications. One of the most promising is the correction of genetic mutations in patient-derived stem cells. For instance, in diseases caused by a single genetic mutation, such as cystic fibrosis or sickle cell anemia, CRISPR can be used to repair the faulty gene in the patient’s stem cells. These corrected cells can then be differentiated into the desired cell type and transplanted back into the patient, potentially curing the disease at its source.
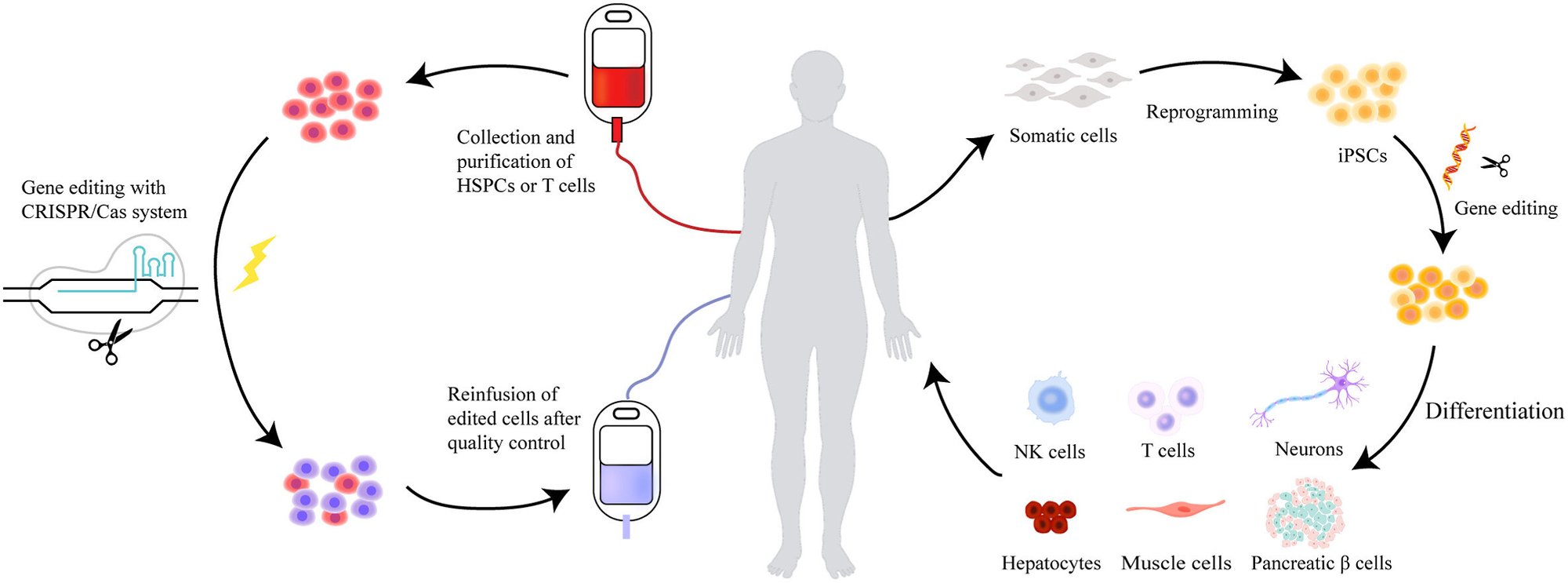
Fig. 2: The use of CRISPR in stem cell therapy. (Credit: Current advances of CRISPR-Cas technology in cell therapy, Cell Insight)
CRISPR is also being used to enhance the properties of stem cells. For example, researchers are engineering stem cells to be more resistant to immune rejection, a major barrier in allogeneic stem cell transplantation. By modifying the expression of certain surface proteins, scientists can create stem cells that are less likely to be recognized and attacked by the recipient’s immune system, increasing the success rates of transplants.
How CRISPR-edited iPS Cells are Transforming Modern Medicine
induced pluripotent stem cells (iPSCs) can be used to generate virtually any cell type in the body, just like an embryonic stem cell. This versatility has made iPS cells invaluable to a variety of scientific fields, including functional genomics, disease modeling, drug discovery and development, cellular immunotherapies, and regenerative medicine. One of the advantages of iPS cells is that they can be generated from somatic cells sourced from patients and collected via relatively noninvasive procedures. In many respects, this eliminates the need to harvest stem cells from human embryos and cuts through a significant amount of ethical red tape.
iPSCs are also more reflective of biological complexity than many of the immortalized cell lines that have typically been used in research, making them a key alternative to these cell types. This is because iPSCs are collected directly from patients, enabling the generation of models and isogenic cell lines with patient-specific genetic backgrounds. With this unique capability, iPS cell technology is shaping the future of what is now known as personalized medicine.
While the impact and applications of iPSCs are manifold, it currently takes long periods and significant expense to generate and grow iPS cell lines for each and every patient that requires regenerative treatment. In this respect, CRISPR is extremely relevant, because it can be used to easily and precisely edit the genome of iPS cell lines that can be used therapeutically in any patient, eliminating the need to harvest cells from each individual and speeding up the process of preparing tailored treatment. CRISPR interference (CRISPRi) can also be used as an alternative strategy to reprogram iPS cells.
CRISPR iPS cells are also transforming the landscape of both preclinical research and clinical trials. By enabling the creation of precision models of human disease, iPSC gene editing allows researchers to become less reliant on animal models. This helps to accelerate the entire clinical development process, saving valuable time and resources and allowing studies to progress into clinical trials.
Editing iPSCs is Not Easy
To the surprise of many scientists, however, using these older gene-editing techniques to edit iPSCs proved difficult. Put simply, iPS cells don’t like to be genetically manipulated. Early iPS cell editing experiments were often inefficient or unsuccessful, not to mention extremely time-consuming.
One of the factors contributing to the resistance of iPSCs to genetic manipulation is that homology-directed repair (HDR) occurs even less frequently in iPS cells compared to immortal cell lines. Attempts to knock-in genes or correct mutations in iPS cells were largely unsuccessful because HDR was not occurring at high enough rates to repair the double-stranded breaks caused by CRISPR-Cas9. Performing multiple edits in iPSCs, which is often necessary to create models of multifactorial inheritance disorders, is even more challenging.
Overcoming Stem Cell Research Challenges with EditCo
EditCo’s automation capabilities ensure high editing efficiencies while maintaining a high standard of quality across iPS cell line development. In partnership with our downstream service providers, EditCo can provide end-to-end services for iPS cell line development (editing to differentiation).
Intelligent Guide Design

EditCo’s innovative approach to smart guide design allows efficient gene disruption generation, eliminating the trial and error associated with common guide design strategies. All our reagents are designed to streamline your research, ensuring more accurate and reliable results.
Better CRISPR Reagents for Efficient, Consistent Gene Knockouts
With intelligent guide design strategy, EditCo is able to simply and effectively knockout genes guaranteed with our Gene Knockout Kits or do high-throughput genomic screening with Arrayed gRNA Libraries, allowing for clear genotype-to-phenotype answers for many genes at scale and quickly.
Outsourcing iPS Cell Editing for Better Reliability and Functionality
Especially if you don't have the time, equipment, or lab capacity to create your own CRISPR iPS cells. Fortunately, EditCo can help! Leveraging our automation capabilities, our workflow for generating CRISPR iPS cells includes several steps that ensure high editing efficiencies while maintaining a high standard of quality.
As seen in the illustration below, EditCo employs a streamlined workflow that can produce pools and clones of edited iPS cells. Edited pools are cell populations that contain both edited and wild-type cells. Clones are populations of genetically identical cells that contain the desired edit.

Experience Unmatched CRISPR Knockout Efficiency in iPSCs
Discover the one-stop solution for reliable and precise knockouts in induced pluripotent stem cells (iPSCs) with EditCo's Engineered Knockout iPS Cells. Our advanced CRISPR editing technology ensures consistently high knockout efficiency (>95%), achieving near-complete functional knockouts even before clonal selection. Utilizing high-quality synthetic non-integrating reagents, our proprietary automated process maintains cell viability and pluripotency, ensuring your cells are artifact-free and your experimental integrity remains intact. Choose EditCo’s Engineered Knockout iPS Cells, available as 100% knockout guaranteed clones or as efficient, non-sorted pools, to elevate your gene function and disease linkage studies. Unlock unprecedented potential and accelerate your research with EditCo.
Have more questions? Reach out to us!
