CRISPR-edited Knockout iPS Cells
Accelerate disease model development, drug discovery, and regenerative medicine with the high editing efficiency and precise genomic integration of EditCo's edited iPS Cells.
Accurate, efficient models for advanced disease research.
-
Exceptional Quality: iPSC quality, pluripotency, and cell integrity are maintained through EditCo’s editing process.
-
Unparalleled Editing Efficiency: Our automated, optimized platform, superior guide design technology, and quality reagents result in high-efficiency editing.
-
Assay with Confidence: EditCo’s robust CRISPR editing process consistently delivers functional gene knockouts for confident assay results.
Experience Unmatched CRISPR Knockout Efficiency in iPSCs
Discover the ultimate solution for reliable and precise knockouts in induced pluripotent stem cells (iPSCs) with EditCo's Engineered Knockout iPS Cells. Our advanced CRISPR editing technology ensures consistently high knockout efficiency (>95%) while maintaining cell viability and pluripotency through our proprietary automated process.
Elevate your gene function and disease linkage studies with EditCo and accelerate your research potential.
XDel Knockout Cell Pools and Clones
High-Performance Knockout Cells for Breakthrough Discoveries
XDel is a cutting-edge CRISPR knockout technology designed to remove the guesswork from gene editing. By leveraging an innovative guide RNA design strategy, XDel delivers highly efficient, consistent, and reproducible gene knockouts—empowering researchers to accelerate their discoveries with confidence without compromising pluripotency and cell integrity.
Whether you're conducting gene function studies, disease modeling, or drug development, XDel ensures robust results that drive meaningful insights.
- High Efficiency: Achieve superior on-target editing rates and consistent knockout performance
- Reliable Results: Experience persistent protein depletion, validated through functional assays
- Reproducibility: Minimize variability for dependable outcomes across experiments in any loss-of-function study
Features
Cell Source
- EditCo supplied (see Cell Lines below)
- Customer supplied
Available Edits
- Indel (standard) or fragment deletion
- Homozygous (standard) or heterozygous edits
- Cell pools and clones
CRISPR Design
- Synthetic modified sgRNA (standard)
Add-Ons
- Additional clones
- QC: Pluripotency testing
- QC: Karyotype Testing
Deliverables
- Regular updates on your order's progress
- Edited cell pools (2 vials with 5x10⁵ cells/vial) or 2 independent clones with the required knockout (2 2 vials with 5x10⁵ cells/vial)
- Mock-transfected cell pools (2 vials with 5x10⁵ cells/vial)
- Sequence of synthetic sgRNA used
- Primer sequences used for NGS sequencing
- NGS sequencing analysis report for each edited pool after expansion.
- Comprehensive QC report that includes the following information: mycoplasma test (positive/negative), passage number, and analysis for add-on QC
XDel delivers higher and more consistent on-target editing compared to single-guide, ensuring superior editing performance
While traditional single-guide knockouts demonstrate a wide and often unpredictable range of on-target editing efficiencies, EditCo XDel technology stands out for its consistent high performance. XDel ensures reliable outcomes across many different cell lines, often outperforming single-guide in both efficiency and reproducibility.

Figure 1. XDel multiple gRNA on-target editing efficiency is significantly higher and more consistent compared to single-guide RNA methods. On-target editing efficiency of multi-guide (pink bars) vs their 3 respective single-guide RNAs (blue bars) across 7 endogenous target loci transfected in two immortalized (IMM) and two iPSC cell lines indicates that XDel technology delivers significantly higher and more consistent on-target editing efficiency compared to single-guide. Each transfection was performed in triplicate and data were analyzed via NGS by EditCo’s proprietary software analysis.
XDel technology maximizes on-target gene editing while minimizing off-target effects compared to single-guide editing
Unlike single-guide RNA strategies, which show variable editing efficiencies across different doses, EditCo’s XDel technology consistently achieves high on-target editing efficiency. This reproducibility highlights the robustness of XDel technology ensuring superior and reproducible editing performance, allowing you to focus on your research without the need for additional optimization.

Figure 2. XDel multiple guide RNA off-target editing efficiency is significantly lower compared to single guide RNA methods. Off-target editing efficiency of XDel cells (pink bars) at all 3 of their respective single-guide off-target sites vs their 3 respective single-guide RNAs (blue bars) across 63 off-target loci (3 off-target loci per 7 on-target site tested) transfected in two immortalized (IMM) and two iPSC cell lines indicates that XDel cells deliver significantly lower off-target editing efficiency compared to single-guide. Each transfection was performed in triplicate and data were analyzed via NGS by EditCo's proprietary software analysis.
Robust Knockouts Without Compromising Quality
XDel pools and clones were tested for karyotyping and PluriTest™ to confirm genomic stability and pluripotency.

XDel knockout cell pools show persistent protein depletion, enabling their direct and reliable use in functional assays
Figure 3. Edited cell pools utilizing the multi-guide technology shows persistent protein depletion. This enables direct and reliable use of these cell pools for functional assays. Western blot analyses for all 5 knockout target genes indicate a complete depletion of proteins across the 3 specified time points (days 7, 14, and 21) relative to the negative controls. Knockout cell pools for the target genes were generated by nucleofecting U2OS cells with multi-guide sgRNA and Cas9 (as RNPs). A negative control pool was also transfected for each target using non-targeting sgRNA.
How does the XDel strategy work?
Traditional CRISPR knockout methods often depend on a single guide RNA that, in tandem with SpCas9, generates assorted insertions or deletions (indels) at the target cut site. This approach can be unpredictable, leading to variable edits and incomplete knockouts.
EditCo’s smart informatics generates a multi-guide design which is composed of up to 3 sgRNAs targeting a single gene of interest. The guides are spatially coordinated and work cooperative to induce a guided repair that results in fragment deletion in an early exon, making it the most reliable knockout strategy compared to other pooled strategies.

Figure 4. XDel design includes up to 3 modified sgRNAs (grey bars) that target a single gene of interest. When co-transfected, the sgRNAs create concurrent double-stranded breaks at the targeted genomic locus and consequently induce one or more 21+ bp fragment deletions.
Single-guide RNA Knockout Cell Pools & Clones
Accelerate Your Discoveries with Tailored CRISPR Knockout Cells
For researchers seeking precise, custom-defined edits for sophisticated and specialized applications, EditCo’s advanced single-guide RNA technology delivers unparalleled flexibility and accuracy. Our single-guide solution empowers you to achieve tailored gene edits with ease:
- Precision: Focus edits on specific regions to achieve the intended functional impact without compromising performance or efficiency
- Custom-Defined: Create bespoke knockouts to suit the specific requirements of your research
- Applications: Ideal for complex research workflows, from disease modeling to pathway studies and beyond
Whether you're working with complex disease models or testing new hypotheses, EditCo’s single-guide solution supports your research with the precision and reliability you need to make breakthrough discoveries.
Features
Cell Source
- EditCo supplied (standard)
- Customer supplied
Available Edits
- Indel (standard) or fragment deletion
- Homozygous (standard) or heterozygous edits
- Cell pools and clones
CRISPR Design
- Synthetic modified sgRNA (standard)
Add-Ons
- Additional clones
- QC: Pluripotency testing
- QC: Karyotype testing
Deliverables
- Regular updates on your order's progress
- Edited cell pools (2 vials with 5x10⁵ cells/vial) or 2 independent clones with the required knockout (2 2 vials with 5x10⁵ cells/vial)
- Mock-transfected cell pools (2 vials with 5x10⁵ cells/vial)
- Sequence of synthetic sgRNA used
- Primer sequences used for NGS sequencing
- NGS sequencing analysis report for each edited pool after expansion.
- Comprehensive QC report that includes the following information: mycoplasma test (positive/negative), passage number, and analysis for add-on QC
Validate your Assays with up to 95% Protein Knockout
EditCo’s Knockout Cell Pool gives you the protein knockout you need in the cell line you want, enabling you to either assay pools directly or quickly move to isolate single cell clones.
Figure 1. EditCo’s Knockout Cell Pool achieved 95% protein knockout without clonal expansion. A cell pool with a surface protein knocked out was generated in U2OS cells with a Knockout Score of 87% (left panel, which is the percentage of indels that either introduce a frameshift mutation or are larger than 21 nucleotides, analyzed by ICE). Levels of this protein were analyzed by flow cytometry (blue) and were reduced by 95% relative to wild type (green, right panel). Negative control samples were stained with an isotype control antibody (purple). Data provided by Eurofins-DiscoverX.
EditCo Delivers Pluripotent Edited Cells
Quality comes first in the development of EditCo’s Knock-in iPS Cells by using our automated workflow and transient RNPs to maintain pluripotency of your cells.

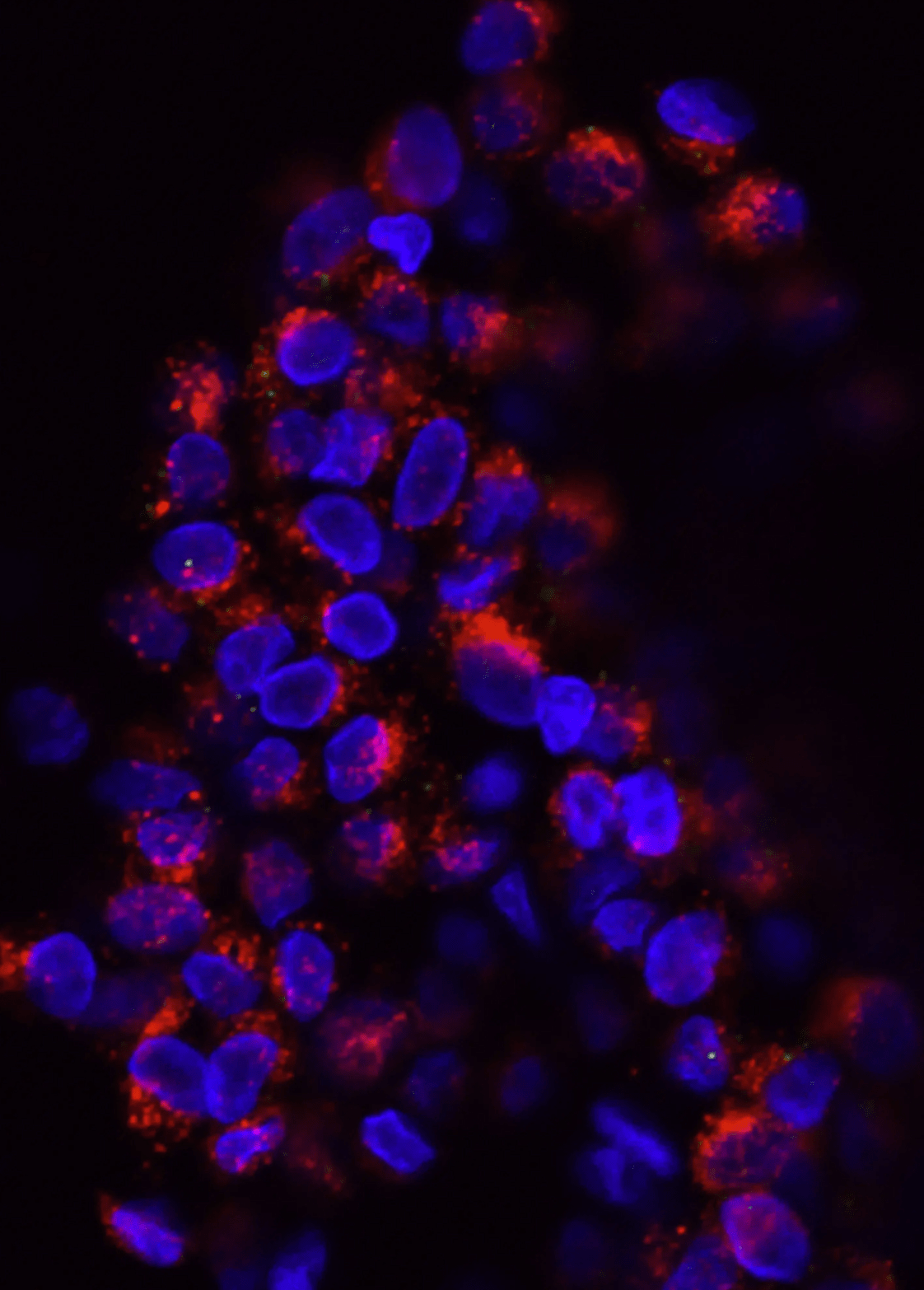
Figure 3. iPS cells were assessed for standard pluripotency markers, three days post-editing.
Use Our iPS Cell Lines or Onboard Your Own
EditCo-supplied cell lines available for all engineered iPS cell orders at no additional cost

* Parental vials available for evaluation prior to booking an edit
Unprecedented Knockout Editing Efficiency
EditCo’s Knockout iPS Cells deliver reliable protein knockout enabling your gene function studies.
Figure 1. Modified gRNAs were transfected along with Cas9 as RNPs to knock out RELA in four individual iPS cell lines. Each population of cells was PCR-amplified around the cut site, Sanger-sequenced, and submitted for ICE analysis to determine editing efficiency.
Everything you need to get screening with Speed and Confidence
EditCo’s iPSC Knockout Libraries simplify high-throughput CRISPR screening for functional assays, overcoming challenges like complex differentiation protocols and transfection inefficiencies.
Our knockout iPSC pools, designed with advanced XDel guide design technology, achieve over 90% editing efficiency and cell viability and come in a ready-to-use 96-well arrayed format. With streamlined workflows, comprehensive QC reports, and compatibility with diverse downstream assays, these libraries enable robust target discovery and validation—so you can focus on your research while we handle the CRISPR complexities.
The iPSC Knockout Libraries workflow includes XDel guide design, high-throughput transfection, knockout pool generation, genotyping, and sequencing analysis, ensuring efficient and precise CRISPR editing at scale.
Resources
Integrating our core CRISPR expertise, high-quality reagents, and automated processes, we deliver the best edited cell-based models at any scale.

Use cases diam faucibus donec pretium leo risus commodo sit. Phasellus a sit.



Quisque amet eu viverra aliquam sed. Montes nulla nisi sit vel urna leo. Nisi donec consectetur bibendum tortor. Gravida arcu lorem venenatis nulla sodales quis sed.
Testimonials consectitur amet sed
Congue fermentum eros leo commodo. Integer vitae vitae ac amet. Vitae sed lectus vel lacus adipiscing nulla facilisis mauris porttitor. Viverra elementum odio vitae id.

Viverra aliquam volutpat interdum quisque vel mattis. Malesuada aliquam egestas pharetra a tempus ullamcorper egestas.

Nibh luctus pulvinar sagittis faucibus commodo vitae purus. Ullamcorper dictum.

Congue fermentum eros leo commodo. Integer vitae vitae ac amet. Vitae sed lectus vel lacus adipiscing nulla facilisis mauris porttitor. Viverra elementum odio vitae id.

Congue fermentum eros leo commodo. Integer vitae vitae ac amet. Vitae sed lectus vel lacus adipiscing nulla facilisis mauris porttitor. Viverra elementum odio vitae id.

Congue fermentum eros leo commodo. Integer vitae vitae ac amet. Vitae sed lectus vel lacus adipiscing nulla facilisis mauris porttitor. Viverra elementum odio vitae id.

Lectus Pluristyx eu vel leo lectus mi. Viverra cursus neque eget proin.
Pellentesque nec tempus ultrices purus amet adipiscing aliquam. Posuere imperdiet adipiscing non blandit non. Sed eros vel sodales magna sem tincidunt orci risus. Molestie hendrerit cursus faucibus ac neque eget turpis. Arcu leo risus iaculis mi. Tortor bibendum sed cras pretium leo.
0+
0+
0+
Lorem Ipsum Dolor Heading
Etiam sollicitudin, ipsum eu pulvinar rutrum, tellus ipsum laoreet sapien, quis venenatis ante odio sit amet eros. Duis arcu tortor, suscipit eget, imperdiet nec, imperdiet iaculis, ipsum. Maecenas nec odio et ante tincidunt tempus. Donec elit libero, sodales nec, volutpat a, suscipit non, turpis. Quisque id mi.
Resources aliquam procte








