Disease Model Development
Developing Disease Models: How CRISPR is Revolutionizing Biomedical Research
Disease models are critical tools in biomedical research, allowing scientists to study the mechanisms of diseases, test potential treatments, and explore disease prevention strategies. Traditional models, whether animal-based or cellular, have provided invaluable insights into human health. However, these models often come with limitations, such as differences in physiology between humans and model organisms, or the difficulty in accurately replicating complex human diseases in a laboratory setting. This is where CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) technology comes into play, offering unprecedented precision and efficiency in the development of disease models, thus pushing the boundaries of what is possible in medical research.
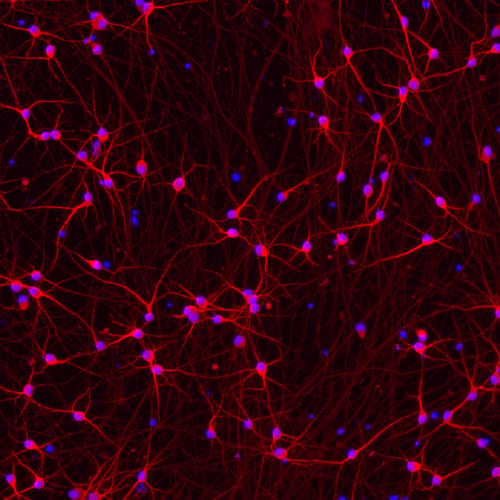
Figure 1. Human iPSC-derived Parkinson's disease model. (Credit: bit.bio)
The Evolution of Disease Models
Traditionally, disease models have included a range of methods, from in vitro systems like cell cultures to in vivo systems like genetically modified animals. Each of these models has its strengths and weaknesses. For instance, mouse models have been extensively used to study human diseases due to their genetic similarity to humans and the relative ease of genetic manipulation. However, mouse models often fail to fully replicate the complexities of human diseases, leading to potential discrepancies between preclinical findings and clinical outcomes.
Similarly, cell cultures offer a more direct approach to studying cellular mechanisms of disease but often lack the context of a living organism, which can limit their utility in understanding how a disease progresses in a complex biological environment. Furthermore, traditional genetic manipulation techniques, such as homologous recombination, can be time-consuming and inefficient, making the development of accurate disease models a slow and labor-intensive process.
CRISPR: A Game Changer in Disease Model Development
CRISPR technology has transformed the landscape of genetic engineering since its introduction in 2012. Derived from a natural defense mechanism found in bacteria, CRISPR allows scientists to make precise edits to the genome by targeting specific DNA sequences. The system uses a guide RNA to direct the Cas9 enzyme to a specific location in the genome, where it can cut the DNA. Once cut, the DNA can be repaired in a way that introduces or corrects genetic mutations, effectively allowing for the "editing" of the genome.
This level of precision and efficiency has made CRISPR an indispensable tool in the development of disease models. Researchers can now introduce specific genetic mutations into cell lines or animals that are known to cause diseases in humans, thereby creating models that closely mimic the human condition. This ability to replicate the genetic basis of diseases in a controlled environment is crucial for understanding the underlying mechanisms and for testing potential therapies.
.png?width=800&name=unnamed%20(84).png)
Figure 1. Animal models help researchers understand genetic diseases. When searching for DNA sequences that cause disease, researchers compare DNA from diseased and healthy individuals. To determine if one of these candidate sequences causes the pancreas defect, researchers will put them into model organisms. In this case, the model organism should be biologically similar to a human, have a copy of the same gene, and should have the same organ of interest. Here, a mouse is a good choice. Researchers can use CRISPR tools to put the candidate DNA sequences into separate mice. If one of the mice develops the defect, it’s likely the defect is caused by the associated DNA sequence. Later, researchers can use these same mice and other model organisms with the “disease DNA” to test potential treatments. (Credit: Innovative Genomics Institute)
CRISPR in Animal Models
One of the most significant applications of CRISPR in disease model development is in the creation of genetically modified animal models. For example, CRISPR has been used to develop mouse models of various human diseases, including cancer, neurodegenerative disorders, and cardiovascular diseases. By introducing precise genetic mutations that are associated with these conditions in humans, researchers can study how these mutations lead to disease in a whole organism, providing insights that are difficult to obtain from cell cultures alone.
Moreover, CRISPR has enabled the creation of animal models that were previously difficult or impossible to generate. For instance, large animal models like pigs, which are more physiologically similar to humans than mice, can now be genetically modified with relative ease using CRISPR. This has opened new avenues for studying diseases such as cardiovascular disorders, where the anatomy and physiology of the animal model are critical to understanding the disease.
CRISPR and Humanized Models
Another exciting application of CRISPR in disease model development is the creation of humanized models. These are animal models that carry human genes, tissues, or even entire organs. For example, researchers have used CRISPR to replace mouse immune system genes with their human counterparts, creating a model that more accurately mimics the human immune response. This is particularly valuable in studying diseases like HIV, where the interaction between the virus and the human immune system is crucial to understanding disease progression and treatment responses.
CRISPR in Cellular Models
CRISPR is also revolutionizing the development of cellular models of disease. Human cell lines, particularly induced pluripotent stem cells (iPSCs), can be edited using CRISPR to carry specific disease-associated mutations. These cells can then be differentiated into various cell types, such as neurons or cardiac cells, which are relevant to the disease being studied. This approach allows researchers to study the effects of genetic mutations in a human cellular context, providing a more accurate model of the disease than traditional cell lines.
Furthermore, CRISPR-based gene editing has facilitated the development of isogenic cell lines, where only one specific gene is altered while the rest of the genome remains unchanged. This allows for direct comparison between mutant and wild-type cells, helping to pinpoint the exact effects of a particular genetic mutation on cellular function.
How EditCo is Solving Challenges in Disease Modeling
CRISPR Engineered Cells Enable Variant Disease Modeling

Cell-based models are elemental to preclinical disease research. These systems are essential to study disease biology and inform therapeutic targeting. Most genetic diseases are complex and heterogeneous, with multiple genetic variants in the population. General lab workflows are often limited by resources and technical inconsistencies in exploring a wide range of variants.
Simplify Building Gene Knockouts with Edited-to-Order Engineered Cells
An option is EditCo’s advanced cells for hassle-free CRISPR knock-ins, such as SNPs, deletions, and point mutations, in any immortalized cell lines, iPS cells, and primary cells. Specify your cell line and desired knock-in, and EditCo’s robust design and editing process will get the job done. No CRISPR optimization on your end required. Doing CRISPR yourself is also easy with EditCo’s guaranteed Gene Knockout Kits.
EditCo solves these problems by generating disease models that include genotypic variants and multiple clones using automation at scale. Our cell engineering platform leverages automated and highly standardized processes for consistent editing and delivers robust CRISPR Engineered Cells. EditCo’s Engineered Cells enable scientists to achieve desired genotypes and high-quality cell models (a panel of multiple variants and clones) at an affordable cost. These cell model systems are available at scales accessible to all researchers, which they can then directly utilize for functional assays. Read more about EditCo’s Engineered Cells in disease modeling in our Application Note: CRISPR Engineered Cells Enable Variant Disease Modeling.

The Challenge of Disease Modeling in Primary Cells and Stem Cells
In vitro disease modeling is useful for understanding the genetic basis of a disease, which can lead to the development of better therapies. Primary and stem cell models are becoming more popular than immortalized cell lines for disease modeling, as they are a step closer to the model organism. They provide physiologically relevant results and are increasingly being seen as crucial cell types for generating therapeutics and conducting basic research on cellular pathways. However, primary and stem cells are notoriously hard to handle, making their use in research difficult. Therefore, immortalized cell lines remain vital for modeling diseases, as they are an easier system to manipulate. Cancer studies, in particular, leverage immortalized cell lines, which often originate in tumor samples and can provide a good baseline model for tumorigenesis.
Have more questions? Reach out to us!
